Fortunately I happened to have a half decent camera to take a picture of this inchworm, which is really a kind of caterpillar. I naturally went outside and released it into a bush to munch on some leaves. I admit it, I'm a buggist. If it had been a cockroach crawling up my jacket I probably would have gotten all eeky, smashy, stompy, killy; but I find inchworms, and especially the way they move, kind of endearing.
The scientific name of the family to which the inchworm belongs, and the moth it eventually turns into, is "Geometridae". It comes from Greek, meaning earth-measure. So does the word "geometry". It seems like a peculiar way to segue into a topic on chemistry, but chemistry is called the "central science" for a reason. This is a post on geometry, and how chemistry isn't just about the elements.
As you probably know, unless you live in a box or suffer from severe urbanitis, when caterpillars turn into moths or butterflies, they have to construct a cocoon. Cocoons have been used by humans for thousands of years, primarily to make silk and stay young. While I haven't heard of anyone making clothing from the silk of an inchworm, silks all serve the same function aside from being pretty: They protect the developing insect. Insect silk isn't as renowned for it's strength as that of their arthropod cousins, the arachnids; specifically spiders. However it's the same principles give both kinds of silk such high tensile strengths. The secret lies in their geometry.
When I had an argument recently with someone about vaccination, I mentioned that geometry is a major factor in how chemistry works. People who haven't gone beyond a sophomore organic chemistry class know what I'm talking about. It's often not enough for certain elements to be present in a compound to start guessing its properties, and this is especially true in biological systems. It's how these elements are arranged that can make all the difference. I cannot overemphasize how important this concept is. There are more than a few rules that organic chemists have generated over the years simply to describe geometries. They have arguments over structures, and expend considerable effort resolving the exact structure of a molecule. Over at the Curious Wavefunction there are posts on peer-reviewed research discussing the structure of hexacyclinol, if you want some idea of what that looks like.
In silks, spider and insect, the high tensile strength is primarily due to what are known as β- pleated sheets of proteins linked together. Proteins are huge molecules. We call them macro-molecules, containing potentially thousands of atoms. Beta-pleated sheets, as the name implies are arranged like the pleat of a skirt. This arrangment offers crystalline strength. The protein "threads" that link the sheets together are also responsible for strength. The precise way that these geometries interact to make silk so strong is something still not completely understood. A lot of work goes into the process of first discovering the precise arrangements of atoms in the large molecules that comprise silk, and then discovering how this arrangement translates into strength.
The proteins that make up these silks are on the elemental level: nitrogen, oxygen, hydrogen, and carbon (basically). Yet those very things could also be in a racing fuel. To start giving credit to these elements for the strength of silk based on their elemental forms would be ludicrous. It has a lot more to do with the how of elements bonding than the what.
Sometimes what may sound like minor variations in a chemical's structure actually amount to quite a lot. The best example of this is enantiomers. Look at the picture below:
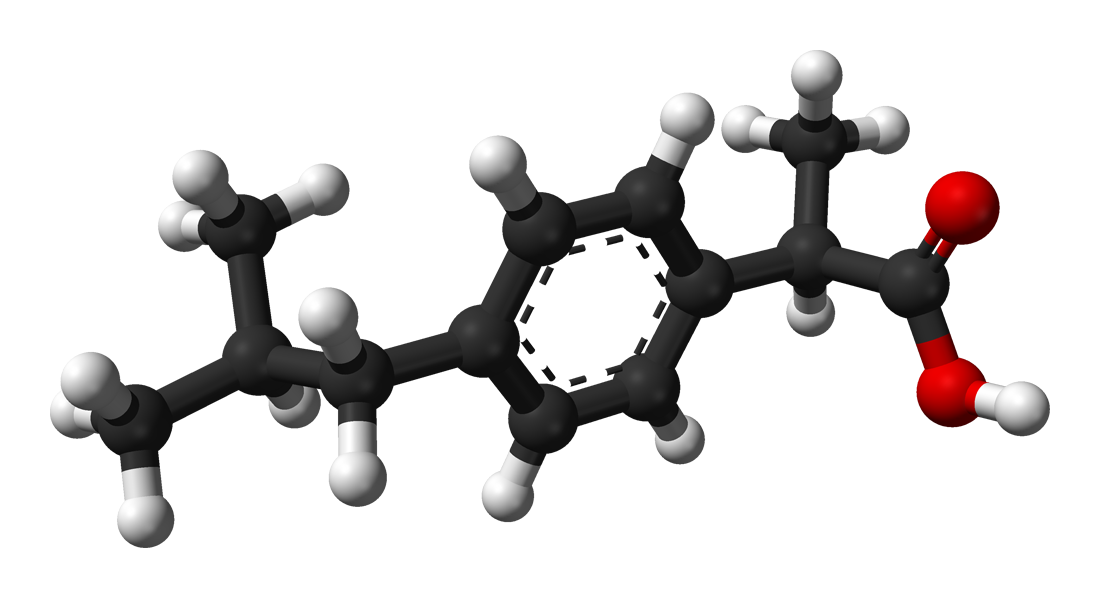
Ladies and gentlemen, meet ibuprofen, perhaps better known to you as Advil or Motrin. It is your best friend when you have a headache. Or is it? Actually that is a picture of (R)-ibuprofen, the right handed form of the molecule. What you want is actually this guy:
 They are literally mirror images of each other. However, only one really cures your headache fast. The (S)-ibuprofen (left-handed form) is the one that acts quickly to cure your headache. The right-handed form kind of screws around in your body a bit before actually turning into the left-handed form. You may not see how they are mirror images, but it becomes clear when you construct a model of them and hold them side by side. These molecules are chiral, the word "chiral" coming from the Greek word for "hand". Your hands are chiral objects. When you hold your hands in front of you, they are mirror images of each other, but if you set one hand on top of the other, the thumbs are at opposite ends. They are in effect, non-superimposable mirror images of each other. We call these enantiomers of each other. They have the exact same boiling point and melting point, but in your body, which is a chiral environment, they have different effects. Chirality is a big deal in biology. In fact, there is some evidence that the right-handed form of ibuprofen may actually slow down the action of the left-handed form. Enantiomers can be expensive and difficult to separate, though. This is why the ibuprofen you buy at the store is actually a mixture of these two forms. For now.
They are literally mirror images of each other. However, only one really cures your headache fast. The (S)-ibuprofen (left-handed form) is the one that acts quickly to cure your headache. The right-handed form kind of screws around in your body a bit before actually turning into the left-handed form. You may not see how they are mirror images, but it becomes clear when you construct a model of them and hold them side by side. These molecules are chiral, the word "chiral" coming from the Greek word for "hand". Your hands are chiral objects. When you hold your hands in front of you, they are mirror images of each other, but if you set one hand on top of the other, the thumbs are at opposite ends. They are in effect, non-superimposable mirror images of each other. We call these enantiomers of each other. They have the exact same boiling point and melting point, but in your body, which is a chiral environment, they have different effects. Chirality is a big deal in biology. In fact, there is some evidence that the right-handed form of ibuprofen may actually slow down the action of the left-handed form. Enantiomers can be expensive and difficult to separate, though. This is why the ibuprofen you buy at the store is actually a mixture of these two forms. For now.This post started with an inchworm and worked it's way into something much longer than I intended at first. It's just a sneak peek into the world of chemistry, it's my way of showing that it's more complicated than throwing two things together and getting a reaction. It's become my best attempt to date at expressing some of the complexity inherent in chemistry in layman's terms. I think it's important people understand that chemistry goes beyond the periodic table, and has depths that they may not be familiar with. I only hope it's been written clearly enough.


Love this post. I'm a Clinical Nutritional Science major & study more so this type of chemistry and the opposite of the type of chemistry studied by chemists.
ReplyDeleteHowever, I do want to learn all chemistry since i do believe that many things in our environment other than just the micro and macro nutrients influence our bodies. ex. radiation...
Also! I web-surf frequently and love reading blogs. I would love for you to post more about chemistry. Do you have any suggestions of other blogs with chemistry conversations?
ReplyDeleteWritten clearly enough for me with only high chemistry to understand.
ReplyDeleteAnd speaking of enantiomers reminds me that I've forgotten to take my escitalopram today.
And by high chemistry I do of course mean high school chemistry, not high faluting chemistry.
ReplyDeleteGreat intro to stereochem post. Pharmaceuticals are definitely the easiest way to make it relevant for people.
ReplyDeleteMost people usually go with the thalidomide example, as it's more dramatic, but the ibuprofen one works as well.
I saw that critter on my lab-mate's shirt the other day. Seems they are finally invading us.
ReplyDelete@ Wuthering, I'm a little mystified about what you mean by radiation. Obviously I get that certain kinds of radiation can be harmful, but I don't see how it enters into most people's daily lives.
ReplyDeleteAs for good chemblogs- Check the blogroll! Other good brainfood abounds in there. Chemistry-blog in particular tends to function as a bit of a hub for chemical blogs. The only thing is that part of the reason I started this blog was because I couldn't really find a lot of chemistry blogs for non-chemists. There are a few of course, Molecule of the Day, for example.
Astronomers and physicists have hundreds of well-known bloggers demystifying their sciences. The biologists, engaged in a (righteous) war with creationism have really gotten the hang of it. The problem with chemistry is that it seems to be a little more arcane and a little less popular than the other sciences. Something on which I've blogged about before.
There are good- scratch that, great books about chemistry for non-chemists by Joe Schwarcz. This is a series of three books that cover a range of topics.
@The Chemist:
ReplyDeleteYeah, I've read Genie in the Bottle by Joe Schwarcz, and it's great stuff, highly recommended.
Hi chemist!
ReplyDeleteI checked out the blogs and love them. I went to google and checked out Joe Schwarcz. @ Will thanks for the heads up on "Genie in a Bottle". I ended up ordering that book and "Brain Fuel". Coincidentally he got terrible reviews for his nutritional knowledge.
Chemist, you had asked the meaning of radiation. I'm not going to go into theories. Although, they have found that chemical structure may change into unexpected forms during food processing. Steriochemistry is one way to explain that such as the realization that hydrogenation ends up creating too many trans stereoisomers. so...
I guess I want to learn chemistry in comprehensive manner so that I will be a better food scientist.
thanks for the wonderful info.
take care!
^^^ oh yeah! I had read that post around the time you had first posted it. I just went back and re-read it and there was a lot more discussion than i had previously seen. I agree that Chemistry doesn't have the same following as biology & physics. I do think more people will begin to study chemistry b/c of technology. Although, i do doubt people will read chemistry on their free time.
ReplyDeletehai I just arrived this post by the label Organic chemistry. Label is not keyword please use to help us.
ReplyDeletewhatever I like it.
U r blog reader can find organic reaction in mastering chemistry
keep posting